November 2025. Volume 35.
Dermatology Snapshots
Highlights:
A new way of thinking about diagnosing mosaic pigmentary disorders
Nail melanoma and melanonychia - length of monitoring and changes to look out for
Cutaneous immune-related adverse events and oncological outcomes
A Delphi consensus on Topical Steroid Withdrawal
European HS guidelines
FFA in men
Mosaic disorders affecting pigmentation – part 1: how to make a clinical diagnosis
VA Kinsler. Br J Dermatol. 2025;193(5):819-829. doi:10.1093/bjd/ljaf262
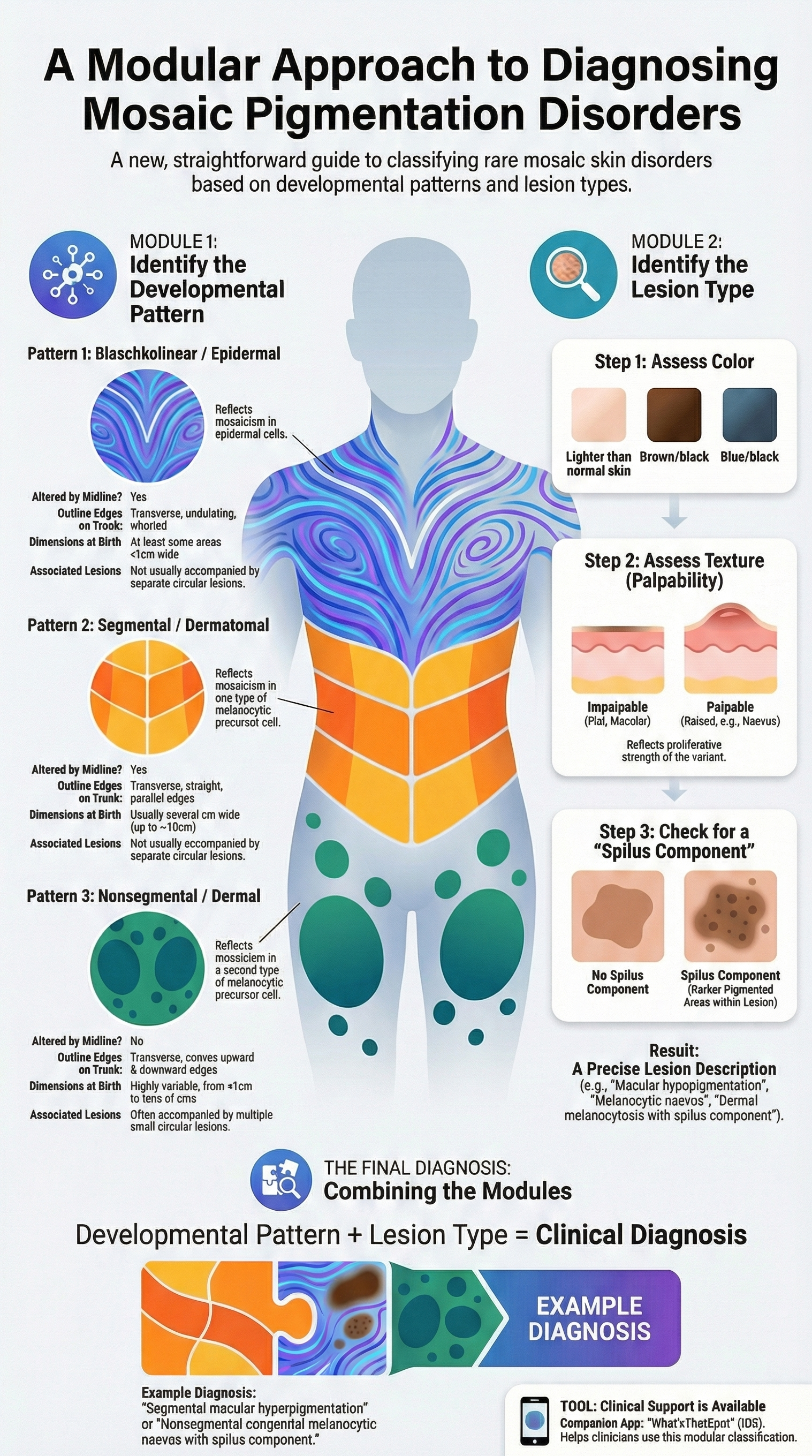
Mosaic disorders (MDs) affecting pigmentation have traditionally been difficult to diagnose due to their rarity and phenotypic variability. This landmark review introduces a modular classification system—linking skin patterns to embryonic cell lineages—to simplify diagnosis and guide management.
1. Redefining "Mosaic" vs. "Developmental" Patterns
While MDs are caused by post-zygotic DNA variants that cannot be inherited, the presence of a pattern does not always guarantee mosaicism. Consequently, the review shifts from "mosaic patterns" to "developmental patterns". It identifies three primary recurrent patterns that correlate with specific cell origins:
Blaschkolinear (Epidermal): Characterized by fine, whorled lines (narrower than 1 cm at birth) that respect the midline. This pattern is associated with keratinocyte lineage variants.
Segmental/Dermatomal (Melanocytic Type 1): Large, quadrilateral blocks with a vertical midline cutoff, often following dermatomes.
Nonsegmental/Dermal (Melanocytic Type 2): Roughly circular or ovoid lesions that are not affected by the midline and often appear symmetrical across the trunk10101010.
2. The Modular Classification
Diagnosis is achieved by combining the Developmental Pattern with the Lesion Type (color, palpability, and the presence of a "spilus component").. This unified framework includes everything from macular hypopigmentation to palpable melanocytic nevi, suggesting that the "thickness" of a lesion reflects the proliferative strength of the genetic variant rather than a different disease process.
3. Clinical Application & Tools
Clinicians must distinguish these from germline disorders or "second-hit" scenarios which require different genetic counselling. To assist in real-time diagnosis, a free smartphone app, "What’sThatSpot," has been developed to guide users through this algorithmic classification.
By streamlining these complex presentations into a modular genotype-phenotype system, dermatologists can better predict systemic risks, provide accurate counseling, and prepare for future targeted therapies.
Digital dermoscopy follow-up for acquired longitudinal melanonychia
Moscarella E, Brancaccio G, Apalla Z, et al. J Eur Acad Dermatol Venereol. 2025;39:1955–1960. doi:10.1111/jdv.20822
Why we chose this paper?
Longitudinal melanonychia (LM) is a common but anxiety-provoking presentation, where the balance between avoiding unnecessary nail matrix biopsies and not missing early nail unit melanoma is particularly challenging. While expert opinion has long supported short-term dermoscopic monitoring in equivocal cases, robust data on what constitutes meaningful change over time have been lacking. This multicentre European study directly addresses this gap by systematically analysing which clinical and dermoscopic changes during follow-up are most predictive of melanoma, making it highly relevant to everyday dermatology practice.
Study aim and design
This was a retrospective, multicentre study evaluating adult patients with a single, acquired longitudinal melanonychia undergoing sequential clinical and digital dermoscopic follow-up. Sixty-two lesions were included. Lesions were either excised (with histology as the gold standard) or, if not excised, required a minimum of 12 months of documented clinical and dermoscopic stability. Baseline features were compared with follow-up findings to identify changes associated with melanoma development.
What were the main findings?
Median follow-up was 17 months (range 2–88 months).
Twenty-seven lesions were excised; six (9.7%) were diagnosed as melanoma in situ.
At baseline, benign and malignant lesions shared similar features, highlighting the difficulty of early diagnosis.
During follow-up, three changes were significantly associated with melanoma:
Increase in pigmentation intensity (83.3% of melanomas vs 14.3% of benign lesions)
Increase in the number of colours (50% vs 8.9%)
Appearance of granular pigmentation (33.3% vs 3.6%)
Band enlargement alone was less discriminating than qualitative pigment change.
Melanomas were excised anywhere from 2 months to over 5 years after baseline, underscoring variable growth rates.
Limitations and applicability
The number of melanoma cases was small, and all patients were Caucasian and managed in specialist pigment clinics, which may limit generalisability. Only pigmented lesions were included, so findings cannot be extrapolated to amelanotic nail melanoma. Nevertheless, the study provides practical, evidence-based criteria to guide monitoring decisions. Importantly, it supports longer-term monitoring of stable melanonychia, often beyond one year, provided there are no concerning dermoscopic changes.
What’s the take-home message?
Sequential digital dermoscopy is a safe and effective strategy for managing equivocal acquired longitudinal melanonychia. Darkening, new colours or granular pigmentation — rather than width alone — should prompt biopsy, and stable lesions may require prolonged monitoring to confidently exclude melanoma.
Cutaneous Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors for Patients With Advanced Solid Organ Malignancies
O’Reilly D, Murray G, Fitzpatrick OM, et al. International Journal of Dermatology. 2025. doi:10.1111/ijd.70061
Why we chose this paper?
Dermatologists are increasingly central to the care of patients receiving immune checkpoint inhibitors (ICIs). A recurring clinical dilemma is whether cutaneous immune-related adverse events (cirAEs) simply represent “collateral damage” or whether they carry prognostic meaning. This paper was selected because it provides real-world, multidisciplinary data showing that common skin toxicities may act as a clinical biomarker of treatment efficacy—information that directly influences how confidently dermatologists can manage cirAEs while supporting continuation of life-prolonging oncology therapy.
Study aim and design
This was a single-centre retrospective cohort study of adults with stage IV solid organ malignancies treated with ICIs between 2012 and 2020. A total of 278 patients were included, all of whom received at least one cycle of ICI therapy. The investigators compared progression-free survival (PFS) and overall survival (OS) between patients who developed cirAEs and those who did not, using univariate and multivariate Cox regression analyses adjusted for age, sex, cancer type, and prior systemic therapy.
What were the main findings?
cirAEs occurred in 19% of patients (53/278).
The most common cirAEs were pruritus, eczema/dermatitis, psoriasis, and vitiligo.
Patients with cirAEs had significantly longer survival:
Median PFS: 47.3 vs 20.9 months
Median OS: 60.0 vs 26.0 months
cirAEs remained independently associated with improved PFS and OS on multivariate analysis.
Prior systemic anticancer therapy was associated with a reduced risk of developing cirAEs.
Most cirAEs were grade 1–2 and managed conservatively with topical therapy and antihistamines.
Limitations and applicability
The study is retrospective and single-centre, with potential under-reporting of mild cirAEs and no adjustment for immortal time bias. The cohort was heterogeneous, including multiple cancer types and ICI regimens. Despite this, the findings are highly applicable to dermatology practice, reflecting real-world management rather than trial-selected populations.
What’s the take-home message?
Common cutaneous irAEs are not merely tolerable side effects—they may signal better oncologic outcomes. Dermatologists should manage cirAEs proactively and confidently, facilitating safe continuation of immune checkpoint inhibitor therapy whenever possible.
Frontal fibrosing alopecia in males: a systematic review
Bai JQA, Gupta S, Donovan J. JAAD Reviews. 2025;6:66–68. doi:10.1016/j.jdrv.2025.08.002
Why we chose this paper?
Frontal fibrosing alopecia (FFA) is still widely perceived as a condition almost exclusive to postmenopausal women, which can lead to delayed diagnosis in men. In clinical practice, however, male patients increasingly present with scarring alopecia affecting not only the frontal hairline but also eyebrows, beard, and body hair. This systematic review was chosen because it brings together fragmented case-based evidence to define the clinical spectrum of male FFA, helping dermatologists recognise and manage this underdiagnosed presentation more confidently.
Study aim and design
The authors conducted a PRISMA-compliant systematic review of MEDLINE, Embase, and CENTRAL databases to identify reports of FFA in male patients. Forty publications were included, comprising case reports and small case series, with data on 362 men. Extracted variables included age of onset, clinical features, symptoms, comorbidities, treatments, and outcomes. The quality of evidence was assessed using Oxford Centre for Evidence-Based Medicine criteria.
What were the main findings?
Mean age at presentation was 57 years, with onset typically in the early 50s.
Hair loss extended beyond the frontal scalp in most cases:
Eyebrows (≈70%)
Beard (≈49%)
Sideburns (≈41%)
Body hair (≈33%)
Symptoms were uncommon; pruritus was reported in 23% and trichodynia in 4%.
Frequent comorbidities included androgenetic alopecia, rosacea, and hypertension.
Treatment responses were generally partial and required prolonged therapy.
Limitations and applicability
Most included studies were case reports or small series, limiting the strength of conclusions and precluding formal treatment comparisons. Reporting of disease duration, severity, and outcomes was inconsistent. Nevertheless, the review provides valuable pattern recognition data that are directly applicable to day-to-day dermatology clinics.
What’s the take-home message?
FFA is a relevant and often overlooked cause of cicatricial alopecia in men, frequently involving facial and body hair. Dermatologists should maintain a low threshold for diagnosis and counsel patients about the need for early recognition and long-term management.
Topical Steroid Withdrawal (TSW) Syndrome: Developing Diagnostic Criteria Through a Modified Delphi Method
Hsu C, Guo L, Adams D, et al. Br J Dermatol. 2025; advance online publication. doi:10.1093/bjd/ljaf518
Why we chose this paper?
Topical steroid withdrawal (TSW) is increasingly encountered in clinic, yet remains controversial, inconsistently defined, and often conflated with severe atopic dermatitis or rebound flares. This paper is important because it directly addresses a major clinical gap: the absence of agreed diagnostic criteria. For dermatologists navigating complex patient narratives and growing public awareness of TSW, this study provides a structured, consensus-based framework that can be applied immediately in practice.
Study aim and design
The aim was to develop preliminary diagnostic criteria for the erythemato-edematous subtype of TSW in adults and children. The authors conducted a three-round modified Delphi process involving 11–12 clinicians experienced in managing suspected TSW, alongside a literature review and patient advocate input. Consensus was predefined as ≥75% agreement on the importance of individual diagnostic features.
What were the main findings?
The panel agreed on 18 key diagnostic features, spanning history, symptoms, and signs, including:
History of escalating topical corticosteroid use (dose, potency, or frequency)
Morphology and distribution different from the original dermatosis
Severe burning pain and spontaneous neuropathic symptoms (“zingers”)
Diffuse erythema with sharp cut-offs at palms/soles (“sleeve sign”)
Marked skin thickening and wrinkling on extensor surfaces (“elephant skin sign”)
Diffuse flaking/exfoliation (“snow”)
Hypersensitivity to heat, water, fabrics, or movement
Histopathology was found to be non-specific and not diagnostically discriminatory.
Limitations and applicability
This was a consensus study rather than a validation study, with a relatively small, predominantly US-based expert panel. TSW remains a diagnosis of exclusion, and the proposed criteria have not yet been prospectively tested against control populations. Nevertheless, the findings are highly applicable to everyday dermatology practice, particularly in complex eczema referrals.
What’s the take home message?
TSW is frequently raised in consultations. This study provides a practical, clinician-led framework to support diagnosis. Careful steroid stewardship, pattern recognition, and exclusion of mimics are essential while formal validation studies are awaited.
European S2k guidelines for hidradenitis suppurativa/acne inversa – Part 2: Treatment
Zouboulis CC, Bechara FG, Benhadou F, et al. Journal of the European Academy of Dermatology and Venereology. 2025;39:899–941. doi:10.1111/jdv.20472
Background and rationale
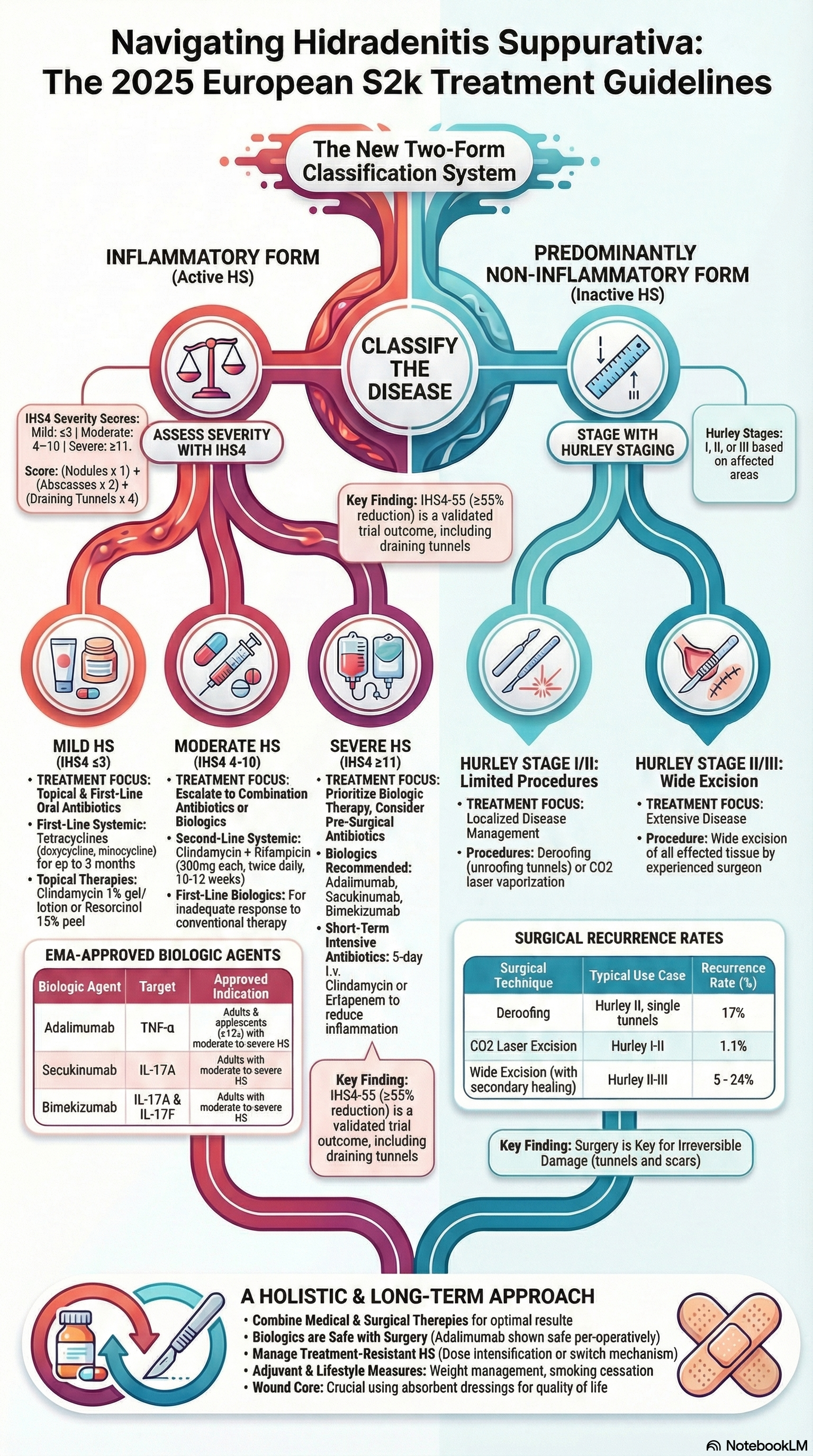
Since the last European HS guidelines (S1, 2015), the therapeutic landscape for hidradenitis suppurativa (HS) has changed substantially, driven by validated severity scoring systems and the emergence of effective biologics. This updated S2k guideline (Part 2) focuses specifically on treatment, aiming to provide dermatologists with a practical, evidence-based algorithm that can be applied across disease severities and care settings.
Guideline development and scope
The guideline was developed using a Delphi consensus process among European HS experts and is based on updated literature and clinical trial data. It emphasises stage-adapted therapy, linking treatment choice to validated severity assessment tools. Importantly, HS is reframed into two dominant phenotypes: an inflammatory form and a predominantly non-inflammatory form, which guides medical versus surgical decision-making.
Key therapeutic recommendations
A central update is the endorsement of the IHS4 score to classify inflammatory HS as mild, moderate, or severe, while Hurley staging guides management of non-inflammatory disease. For mild-to-moderate inflammatory HS, oral tetracyclines are highlighted as first-line therapy, with efficacy comparable to clindamycin–rifampicin combinations and a more favourable safety profile. Short-course 5-day intravenous clindamycin is introduced as a strategy to rapidly suppress inflammation and shorten overall antibiotic exposure.
For moderate-to-severe HS, biologic therapy is now firmly embedded in standard care. Adalimumab, secukinumab, and bimekizumab are all EMA-approved options, with clear dosing and response-assessment guidance. Surgery is recommended for irreversible, tunnel-dominant disease and should be combined with anti-inflammatory medical therapy to achieve optimal outcomes.
Clinical relevance
These guidelines provide a clear, practical treatment algorithm grounded in validated scoring systems and real-world trial data. They strongly support early, adequate therapy and combined medical–surgical approaches to reduce disease progression, scarring, and long-term morbidity in HS.