November 2025. Volume 35.
Dermatology Snapshots
In this edition:
TSPOT test for Mycobacterium marinum
Imiquimod for lentigo maligna - what does the evidence say?
Cognitive biases in clinical practice
Histiocytoid Sweet’s Syndrome - an overview
Deep Dive Slides: Nails and systemic disease & test your knowledge
Application of T-SPOT.TB in the Diagnosis of Cutaneous Mycobacterium marinum Infections and Evaluation of Treatment Efficacy
Cen C, Xie B, Zeng R, Zhang W, Wang H, Sang X. Acta Derm Venereol. 2025;105:adv44119.Why we chose this paper?
Cutaneous Mycobacterium marinum infections are frequently underdiagnosed due to their nonspecific presentation and limitations of traditional diagnostics. This study evaluates whether T-SPOT.TB—commonly used for tuberculosis—may serve as a rapid adjunctive tool for diagnosis and treatment monitoring in M. marinum infection, a topic with growing clinical relevance.
Study aim and design
This retrospective study reviewed 145 culture-confirmed cutaneous M. marinum cases from 2020–2024. T-SPOT.TB was measured at baseline, 3 months, and 6 months after initiation of standard combination antimycobacterial therapy. Outcomes included positivity rates and changes in spot-forming cell (SFC) counts to assess diagnostic value and response-monitoring potential.
What were the main findings?
Baseline T-SPOT.TB positivity: 71% of patients.
At 3 months: positivity decreased from 88.2% to 64.7% (not statistically significant), but median SFCs significantly dropped (20 → 8; p = 0.0007).
At 6 months: positivity decreased from 74.1% to 59.3% (non-significant), with SFCs again showing significant decline (12 → 8; p = 0.0006).
No significant difference in positivity between 3 and 6 months.
Higher baseline SFC values showed the greatest reductions, suggesting immunological responsiveness to therapy.
Limitations and applicability
The study is retrospective, with small follow-up subgroups (n=17 and n=27), which limits generalisability. T-SPOT.TB cannot distinguish M. marinum from tuberculosis, necessitating rigorous TB exclusion. Nevertheless, SFC trends appear promising for adjunctive monitoring.
What’s the take-home message?
T-SPOT.TB is frequently positive in cutaneous M. marinum infections and offers useful immunological trends during therapy—particularly declining SFCs—supporting its role as an adjunct diagnostic and monitoring tool when used alongside standard testing.
Imiquimod 5% for Lentigo Maligna of the Head, Neck and Face: A Systematic Review and Meta-analysis
Ghanem L, et al. J Eur Acad Dermatol Venereol. 2025;00:1–4.Why we chose this paper?
Lentigo maligna (LM) on the head and neck presents unique management challenges due to cosmetic and functional concerns. Imiquimod has become a widely used non-surgical option, but treatment regimens vary considerably. This updated systematic review and meta-analysis focuses specifically on head and neck LM and incorporates new studies, providing clinically relevant clarity.
Study aim and design
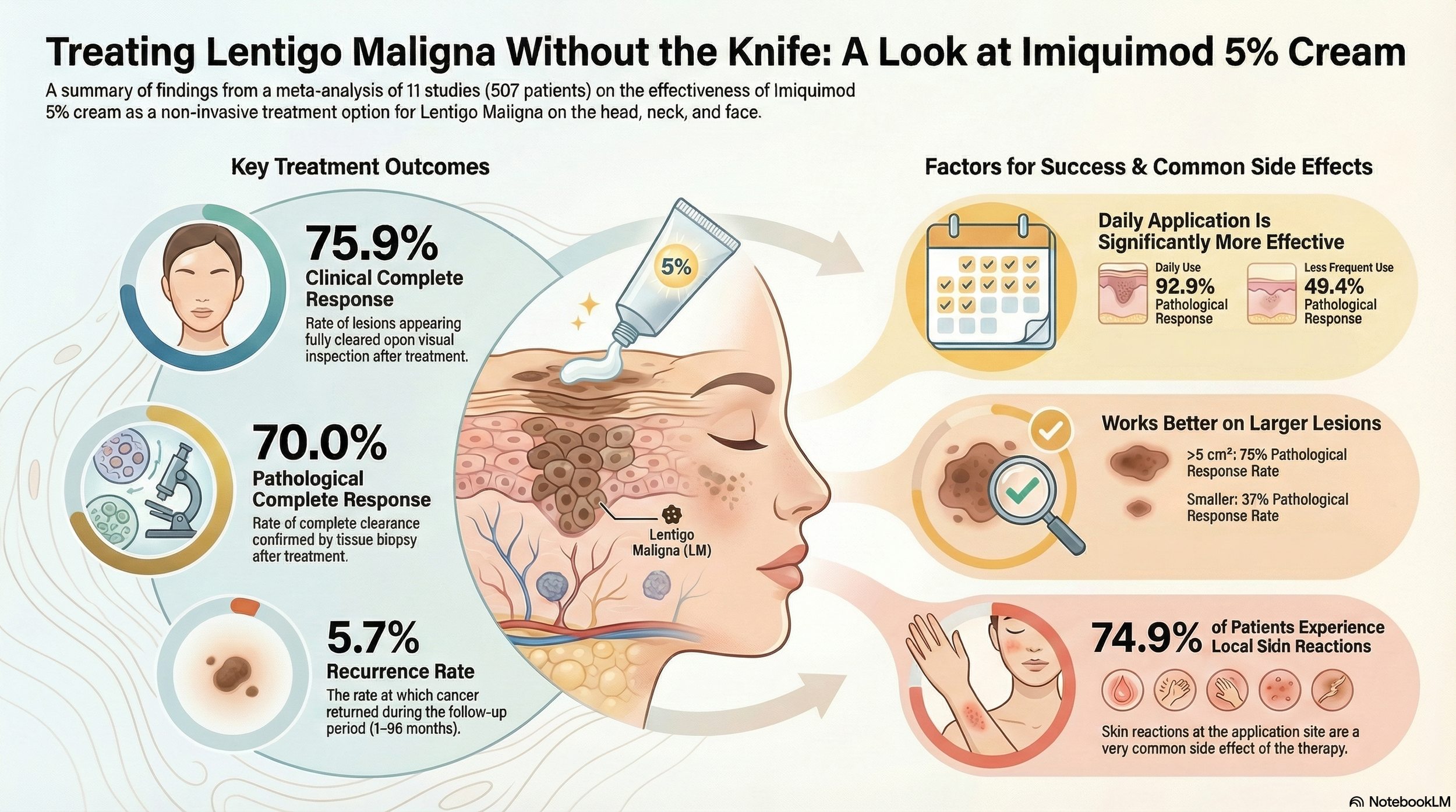
The authors conducted a PRISMA-guided systematic review and meta-analysis of RCTs and observational studies assessing imiquimod 5% monotherapy for LM of the head, neck, and face. Eleven studies (507 patients) were included, with treatment durations ranging from 4–17.7 weeks and follow-up from 1–96 months. Outcomes included clinical complete response (cCR), pathological complete response (pCR), reflectance confocal microscopy complete response (rcmCR), recurrence, and local reactions.
What were the main findings?
Pooled cCR: 75.9% (95% CI 57.3–88.1).
Pooled pCR: 70.0% (95% CI 47.0–86.0).
Larger lesions (>5 cm²) achieved significantly higher pCR (75% vs 37%).
Daily application outperformed less frequent regimens (3-5 times a week) - 93% vs 49%.
rcmCR: 62.7%.
Recurrence: 5.7% across studies.
Local inflammatory reactions: common (74.9%).
No major differences between treatment-naïve and previously treated lesions.
Limitations and applicability
Most included studies were observational with moderate-to-serious risk of bias, substantial heterogeneity, and variable regimens. Long-term recurrence data remain limited, and most studies involved Western populations, limiting generalisability.
What’s the take-home message?
Imiquimod 5% is a promising non-surgical option for head and neck lentigo maligna, with encouraging response rates—particularly with daily application—though higher-quality comparative trials are still needed.
Common things are common: A case study in diagnostic error due to cognitive bias
Anabaraonye N, Wyant WA, Brag K. J Am Acad Dermatol. 2025.Summary
This case report follows a 56-year-old man with a progressively worsening, intensely pruritic eruption initially presumed to be a drug reaction to tirzepatide. Despite discontinuation of the medication and multiple prednisone tapers, his eruption expanded. On dupilumab for months, the eruption evolved into nearly confluent papules with intermittent hemorrhagic crusting. A biopsy eventually showed a clonal CD30⁺ infiltrate, leading to a diagnosis of lymphomatoid papulosis (LyP). Methotrexate was initiated.
However, at the time the patient was scheduled to start phototherapy, clinicians reassessed him and noted widespread crusted plaques and weeping papules. A simple mineral oil preparation revealed numerous scabies mites, eggs, and feces—the true diagnosis. Retrospective review of the earlier biopsy even revealed a single mite leg that had been overlooked. Immunomodulatory therapies likely contributed to the eruption’s severity, including progression toward crusted scabies.
The case highlights how diagnostic error evolves when cognitive biases compound over time. Premature closure, anchoring, familiarity bias, and diagnostic momentum all played significant roles, delaying a simple bedside test that ultimately solved the case. The authors emphasize the importance of maintaining diagnostic humility, revisiting assumptions, and prioritising bedside tools—morphology, scrapings, biopsies—before escalating to targeted systemic therapies.
Cognitive Biases & Practical Tips
1. Premature Closure
Definition: Accepting a diagnosis before it is fully verified, stopping the search early.
How it showed up: The eruption was attributed to tirzepatide with no initial biopsy or scraping.
Practical Tips:
Force yourself to document at least three differential diagnoses before committing.
If treatment fails after a reasonable interval, restart the workup, not the prescription pad.
2. Diagnostic Momentum
Definition: Once a diagnosis is recorded, subsequent clinicians accept it unquestioningly.
How it showed up: The LyP label locked in further decisions—including starting methotrexate and planning phototherapy—despite clinical progression.
Practical Tips:
At each follow-up, perform a fresh first-principles exam: pretend it’s a brand-new consultation.
Before escalating therapy, ask: “Does the current morphology still fit the working diagnosis?”
3. Familiarity Bias
Definition: The tendency to diagnose conditions we commonly see or feel comfortable managing, even when the presentation is atypical.
How it showed up: The eruption was repeatedly interpreted through the lens of familiar entities—drug eruption, eczema, LyP—rather than less common but classic “great masqueraders” like scabies, especially its crusted variant.
Practical Tips:
When a condition looks like something common but behaves unlike it (worsens despite correct therapy, spreads unexpectedly, or shows unusual morphology), pause and seek a disconfirming finding.
Keep a short personal checklist of “conditions I often miss because they look like something else”—scabies, tinea incognito, CTCL, secondary syphilis.
4. Anchoring Bias
Definition: Fixating on the first piece of information or initial impression and failing to adjust when new data appears.
How it showed up: The case anchored on “drug eruption from tirzepatide.” Even as the timeline, distribution, and morphology became incompatible, clinicians continued to interpret each change as a variation of the initial idea.
Practical Tips:
If the morphology outgrows or outpaces your initial hypothesis, force a structured re-evaluation: biopsy, scraping, repeat history.
Use team-based diagnostics—ask a colleague: “What would you call this if you hadn’t read the notes?”
Histiocytoid Sweet Syndrome – A Systematic Literature Review of the Clinical and Histological Characteristics and Treatment Outcomes
J Eur Acad Dermatol Venereol. 2025;00:1–4
Why we chose this paper?
Histiocytoid Sweet Syndrome (HSS) is an uncommon and diagnostically challenging variant of acute febrile neutrophilic dermatosis. It is frequently confused with malignancy-associated dermatoses and myelodysplasia cutis. This systematic review consolidates all available cases and provides valuable insight into its clinical spectrum, histological profile, and important associations with haematological disease and VEXAS syndrome.
Study aim and design
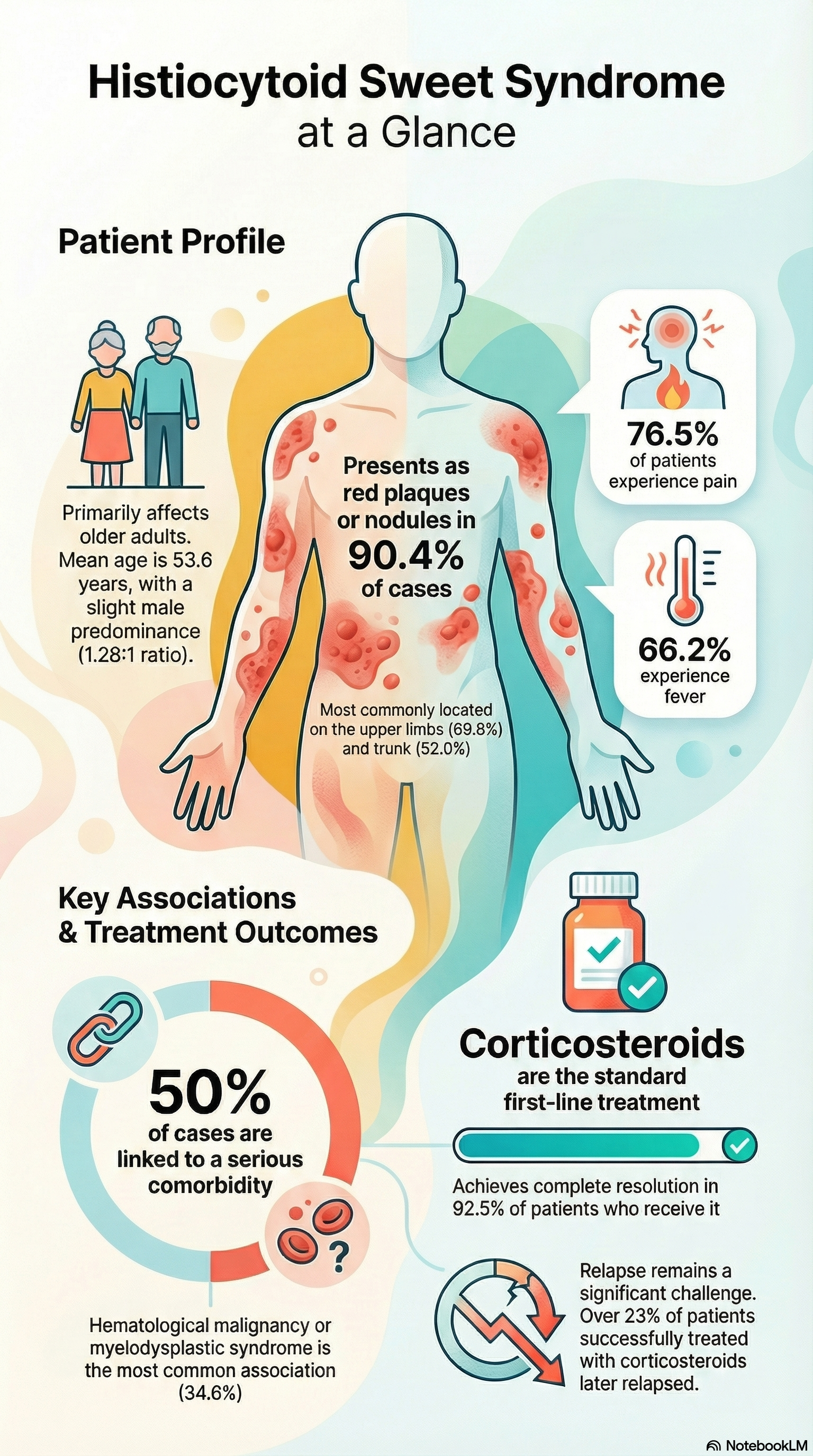
The authors conducted a systematic literature review (Medline, Embase, Cochrane, October 2024) to identify histologically confirmed HSS cases. Paediatric and adult cases were included. Two independent reviewers selected studies. A total of 228 cases across 71 reports (case reports, case series, cohort data) were analysed for demographics, clinical features, triggers, histopathology, comorbidities, and treatment outcomes.
What were the main findings?
Demographics: Mean age 53.6 years; slight male predominance (56%).
Systemic associations:
Haematological malignancy: 19.3%
Myelodysplastic syndrome (MDS): 16.2%
Autoimmune disease: 10.1%
VEXAS syndrome: 7% overall; 35% of cases reported since 2020
Triggers: Drug-induced in 7.5% (notably myeloma therapies).
Clinical presentation:
Erythematous plaques/nodules in 90.4%
Systemic features: fever (66%), pain (76%), arthralgia (25%)
Histology:
Immature myeloid infiltrate with “histiocytoid” appearance
Strong positivity for CD68, CD163, MPO, lysozyme
Treatment outcomes:
Corticosteroids were first-line in 92%; 92.5% complete resolution
Relapse in 23% of steroid responders
Some responded to malignancy-directed chemotherapy, though relapse was common
Limitations and applicability
Data were derived almost entirely from case-based literature, with frequent missing clinical details. MDS-cutis was not used as a search term, likely underestimating its link. Applicability is limited by heterogeneity and lack of prospective data. Despite this, the review provides the most comprehensive synthesis to date, highlighting key diagnostic markers and systemic associations.
What’s the take-home message?
HSS is strongly associated with haematological disease—including MDS and VEXAS—and shows excellent initial steroid responsiveness but notable relapse rates. Clinicians should evaluate for underlying marrow disorders, especially in older men.